Energetics: a new scientific main
INTRODUCTION
Modern energetics is based on engines transforming heat potential difference into other forms of energy; moreover this transforming is far from being full. Everybody knows Carno formula for the ideal heat engine. It is considered that this formula predicts maximum possible efficiency for the heat engine and it implicitly contains in itself the principle of growth of entropy - law of degradation of energy. The problem of efficiency growth is solved traditionally - by means of rise in temperature of actuating fluid. Successful attempts of solving this problem differently are known, for example - ammonia-water thermodynamic cycle of Alexander Kalina. The aim of this article - to indicate two more ways of growth of efficiency of thermodynamic cycle of the heat engine. But it`s not a frontal attack - it is an alternative path, qualitative and in a sense knock out for the whole branches of industry.
CONTROL OF ENTROPY
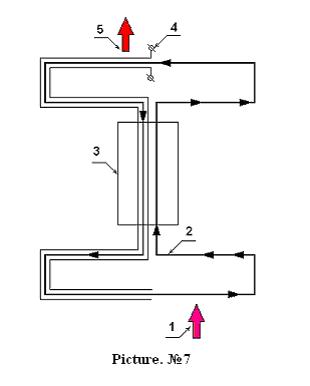
Reports about great success of different research teams in the field of magnetocaloric and electrocaloric effect have appeared in press recently. There exist reports that powders of combinations of gadolinium are heated to 12 degrees under the influence of external magnetic field and they are cooled to the same magnitude when external magnetic field is taken away. There are reports that approximately the same according to the magnitude thermal effect may be attained by means of influence of relatively moderate according to its strength external electric field on films of some substances. There are no doubts that the most suitable substances, i.e. substances with maximal magnetocaloric and electrocaloric effect may be used in the form of fine-dyspersated mechanical solutions and fluid may be used as a simple mechanical carrier for the powder. Using fluids as carriers for mechanical solutions will allow to make use of powders in heat-exchangers and recuperators and introduce powders into zones of influence of external fields easily and bring them out of the influence of external fields easily. Besides even usual liquid solutions have crystal-like structure as in the process of dilution solvation of molecules and ions of solute takes place; some molecular order arises in the process of forming of micelles. It means there exist interesting outlooks in a sense of using in energetics possible thermal effects arising under the influence of external fields on usual and colloid solutions. External fields must organize molecular and quantum structure of solutions, thereby they must decrease chaotic state in solutions so decreasing entropy. Consequently under the influence of external field on the solution it must get warm, cessation of this influence will lead to losing order and cooling and its entropy will grow. Now as an example we can suggest an obvious simplest scheme of recuperation on the basis of electrocaloric effect. See picture N 7.
A closed circuit
in which the mechanical solution of the substance that is able to strong
electrocaloric effect is shown in picture N 7. Circulation of the solution
between upper and lower temperature levels situated in the upper and lower parts
of the picture accordingly takes place in the circuit. Figure 4 points at
clamps of the pair of conductive circuits; these two circuits
surrounding the
flow of the solution form the electric field influencing the powder of the
mentioned substance. A hot solution is fed between circuits 4 where under the
influence of the electric field change of its thermodynamic state takes place
and as a result of this the solution is heated, liberated high-temperature heat
is branched to the place of destination, for example it is transmitted to the
heater of the heat engine. This branching of heat is indicated in the picture
by arrow 5. After decreasing of
temperature of the solution as a consequence of heat emission, it is fed to the
heat exchanger 3 and at the same time influence of external field is not
ceased. When the temperature of the solution in the heat exchanger falls to the
temperature close to the temperature of the lower temperature level, the
solution is taken out of the influence of external field and as a result return
to normal thermodynamic state takes place, the temperature of the solution
falls and as a consequence of this it
becomes possible to lead low-temperature heat indicated by arrow 1 to cold
solution. This low-temperature heat may be branched from the refrigerator of
the heat engine or it may be withdrawn from the external source of
low-temperature heat. Then the low-temperature solution is fed into the heat
exchanger 3 where it is heated to the temperature close to the temperature of
the upper temperature level. Let us pay attention to the fact that
effectiveness of such thermocompressor is rarely high and it is not defined by
Carno limit. It is the first step of the alternative path. But it
is not all; there is a chance to leap farther.
SOLUTIONS AS ACTUATING FLUID OF HEAT ENGINES
To explain the essence let`s take for a start some soluble substance and some solvent. Let this substance dissolve in the mentioned solvent with thermal absorption and accordingly its solubility increases with the rise in temperature. Let`s consider for simplicity that total volume of the substance remains invariable in the process of dilution and heat capacity of the solution is equal to heat capacity of the source solvent and soluble substance. Our interest is fat solutions, i.e. such solutions in which dilution of the given substance at the fixed temperature is no longer possible. We pay special attention to the fact that fat solution is the system that is in dynamic equilibrium, i.e. portion of molecules of the solution passes into the source undissolved substance and equal portion of the substance dissolves at the same time.
In many respects the process of dilution is similar to the process of change of phase such as water evaporation. However it has a number of peculiarities and we`ll briefly mention about them below. The change of phase and dilution are described in classical thermodynamics equally. The difference in Gibbs energy of the substance in concrete phases is considered to be the driving force of both dilution and evaporation. If the liquid strongly boils or the substance strongly dissolves, the solution is not fat and it means there is difference between Gibbs energy of vapor and Gibbs energy of water; for the solution it will be the difference between Gibbs energy of the solution and Gibbs energy of the soluble substance. Now we`ll look narrowly at solutions. In the first place we`ll mention that solutions are characterized by the presence of osmotic pressure that is calculated with the help of gas laws and investigated by Vant Hoff. Posm = RT/V, where Posm - osmotic pressure, R - gas constant, T - temperature, V - volume of solution. If we put a soluble substance into a solvent, first there will be no solution at all, consequently there will be no osmotic pressure for the given solution. Then in the process of dilution a solution of larger and larger concentration will form, osmotic pressure of the solution will increase accordingly. Since in our case (and our interest is this case) dilution will be accompanied by thermal absorption from environment in isothermal conditions then from generally accepted considerations we can say that entropy of the system consisting from the soluble substance and the solvent increases as heat is brought to the solution. On reaching saturation magnitude of osmotic pressure will be a maximum. During such dilution no external work will be performed by the system as the volume of the system consisting from the soluble substance and the solvent remained invariable. All present Gibbs energy is realized as osmotic pressure of the solution. Let`s examine picture N 1.
Here we have the picture of the vessel, which confines the volume of the solvent and soluble substance. There is an ideal mobile membrane that lets pass through only the solvent and it moves without friction. We`ll dissolve the substance on the right of the membrane once again and maintain the process as isothermal.As soon as osmotic pressure arises on the right side, the membrane will begin moving to the left admitting water to the right. Shifting the membrane will reach the left side of the vessel. No work will be performed on the membrane as it can be shifted by infinitesimal superfluous pressure on the right that arises under the influence of osmosis. As a result the membrane will reach the left side when the concentration of the solvent on the right is still minimal. And only later, when the solution becomes saturated, the solution with fixed concentration will occupy the whole volume of the vessel and after that nothing will occur. As it stands the system as a whole will possess some thermodynamic state characterized by the set of thermodynamic values, temperature, volume, concentration, pressure, osmotic pressure, enthalpy. We`ll emphasize that state-of-the-art concrete state of the system is characterized by single concrete value absorbed in the process of dilution heat - Qr.
Now let`s supply
our mobile membrane with the rod and once again carry out the process of
dilution under the same conditions. However now we`ll keep the concentration on
the right at the level of the saturated solution in order to maintain the
highest possible osmotic pressure on the right. With the help of the rod we`ll
receive yield allowing the membrane to move slowly to the left up
to the stop. So all present Gibbs energy
will be spent on making osmotic pressure, consequently dilution heat Qr will
not be enough for performing by the system yield on the rod DA = Posm* DV. The
system will have to take additional heat and transform it into work DQ = DA =
Posm*DV. Starting from canons of thermodynamics it should be expected that
during the process of work the system will have larger entropy in the end then
in the case when it wouldn`t perform yield. The reason of such supposition is
that the system in the second case took more heat from without precisely by the
value that is necessary for performing work on the rod. However finite state
both in the first and second cases will have one and the same operation
factors: the same volume, the same temperature, the same pressure of the system
on the whole and osmotic pressure of the solution, the same concentration - the concentration of saturation. It is obvious that nothing will change if we
permit the piston to move at any osmotic pressure that is less than the osmotic
pressure of the saturated solution. Terminal velocity of diffusion of
molecules, ions and some associations of the soluble substance in the solvent
allows us to accept any level of pressure on the piston. As is well known
diffusion rate depends on temperature, that`s why we can allow the piston to
move making some convenient to us effort. In other words the finite state of
the system will be the same irrespective of work performed by the system and
quantity of heat taken additionally from without. It is interesting, isn`t it?
And at the same time we shall pay attention to that fact, that the machine can work and
with substances which solubility is constant. These substances are dissolved with zero
thermal effect. So the efficiency of the machine in this case in accuracy is equal
to efficiency of Carno.
Let`s consider even more interesting
case. We`ll take some other soluble substance with thermodynamic operation
factors that are identical to the soluble substance examined earlier. Let`s
call it substance N 2. Let it have the same operation factors of dissolubility
as substance N1 - the same heat of dilution in the same temperature span,
consequently the same Gibbs energy and its changes in the process of dilution,
the same thermal capacity of the substances and their solutions and so on.
Therefore curves of dissolubility will be identical. See picture N 3.
In a word we`ll
consider that substance N 2 is completely identical with substance N 1 except
one small detail - substance N 2 forms in the same solvent not usual but
colloidal solution. The peculiarity of colloidal solutions is that the lion`s
share of available Gibbs energy during the process of their formation is spent
on making a very large surface of microdrops of the solution - micelles.
Accordingly osmotic pressure produced by the saturated colloidal solution of
substance N 2 may be many times less than osmotic pressure of the solution of
substance N 1 as in this case osmotic pressure will be produced not by
molecules and ions but micelles which involve a quantity of molecules. In
theory of solutions it is accepted to account for numerical deflection of
osmotic solution from Vant Hoff`s formula to introduce in it a
trial coefficient - i, which was called isotonic coefficient. So for the solution of substance
N 2 Vant Hoff`s formula will be the following one: Posm =
iRT/V, i for substance N 2 will be substantially smaller than one.
All mentioned above arguments for the cases considered earlier both with
receiving yield on the rod and without it retain as applied to colloidal
solution. The only difference is that with the help of the colloid solution
we`ll get less yield on the rod and the system will certainly take up from
outside heater less quantity of heat that is necessary for producing this work.
Now let`s look at picture N 4
Let the solution
of substance N 2 and its colloidal solution be on the left and substance N 1
and its solution (it is a usual solution) - on the right. Let`s have some
supply of undissolved substance of the first and second type on the right and
on the left. Let a mobile membrane be locked in hard with the help of locking
devices as it is shown in the picture. Let the capacity be completely isolated
from the environment. So enthalpy of masses of substances on the left and on
the right, their Gibbs potential are absolutely equal but osmotic pressures of
the solutions are different. Let us see what will happen if we remove locking
devices. Under the influence of larger osmotic pressure on the right the
membrane will start moving to the left. The balance of the saturated colloidal
solution will be upset as the solvent will begin to spill over through the
membrane rightwards. We`ll note especially that energy spent on forming the
surface of micelles is of potential character and completely reversible,
consequently it spontaneously returns when the volume of the solution
diminishes. As a result on the right side of the capacity substance N 2 will
start isolating. All heat liberated in this process will be equal to heat efficiency of
dilution of this substance, plus heat which is numerically equal to work of
surplus osmotic pressure of the usual solution on the right DA = DPosm*DV, here
DPosm = Posm1 - Posm 2. As the
membrane moves forward to the left, portions of pure solvent will come in the
right part. It will give a chance to dissolve for substance N 1 on the right
and drive concentration to saturated state; the quantity of heat exactly equal
to the heat arising in the process of liberation from the solution of substance
N 2 will be required for this. Besides for performing work on the membrane
additional heat exactly equal to the heat liberated during the same work on the
other side will be necessary. In the end when the membrane reaches the stop on
the left, all substance N 2 will be isolated from the solution on the left of
the membrane, to the right of the membrane there will be a saturated solution
of substance N 1 having pulled on its side all the solvent. With
all this going on neither volume nor pressure, nor temperature of the system
will change as the solvent will completely pass into solution N 1. We can
conclude - on the whole the entropy of the system from beginning to end of the
process remains invariable. Such conclusion follows from energy identity of the
solutions and soluble substances according to conditions appointed earlier and
because of the absence of heat exchange with the environment.
The real picture of time history of
the state of solutions and shifting of the membrane will certainly be somewhat
different; temperatures on the left and right sides, their osmotic pressures
and concentrations may differ from the proposed description. Real dynamics
depends on great number of factors: thermal conductivity of substances, the
solution, properties of the membrane and so on. But it is important for us that
at any dynamics in heat-insulated system the finite state of the system will be
characterized by unchanged from the beginning of the process temperature,
absolute pressure, volume and consequently entropy. The only thing that remains
to do is to supply the considered system with the rod in order to receive yield dA = DPosm* dV.
When we begin to receive yield
on the rod, spilling over of the part of available internal energy of
heat-insulated system into yield will take place as the system cannot take
energy for performing work from outside because it is heat-insulated from the
environment. The temperature of the system will certainly drop by the end of
piston stroke, saturated concentration of the solution of substance N 1 will
change and it will become less, the difference of osmotic pressure Posm during
the shifting of the membrane will fall as common temperature of the system
drops, but the volume and absolute pressure after completing the process will
remain invariable.A legitimate question emerges: what changes in principle,
after all the membrane should be returned into primordial state, so it means
that for this it is necessary to apply all obtained work. Everything is true,
but the system should be returned into primordial state at reduced temperature.
It should be cooled for this by leading away heat to some solid; ruse is what
body and we`ll discuss it below. It is evident that in the process of cooling
the system substance N 1 will be isolated from the solution as equilibrium of
the solution will be upset now because of temperature change. Isolation of substance
N 1 from the solution will be accompanied by heat release which should be led
away as well. When required temperature will be achieved, the membrane can be
brought back into reset state. Work spent on it will be substantially smaller
and accordingly quantity of heat that will be necessary to lead away into the
environment is reduced.
So let`s consider thermodynamics of the ideal
osmotic - reverse-osmosis engine. For a start we`ll state the value of it and
later we`ll present the scheme of its work in a closed circuit. In the picture
you can see an assemblage consisting of the cylinder supplied with a mobile
piston the membrane of which is able to pass through only the solvent. The
piston divides the capacity into two parts in which there are substances and
their solutions. Let the heat from the external heater and between parts of the cylinder
be transmitted instantly, thermodynamic process during the movement of the
piston is isothermal, the solutions are in saturated state all the time.
See picture N 5. The initial position of the piston is rightmost.
It is clear to
us that heat of dilution of both substances
- Qr is equal and that`s why they are completely compensated. However
the solution of substance N 1 performs work and additional heat Q1 is necessary
for this. Work is performed on the solution of substance N 2 and it is
necessary to lead away heat Q2 to keep the temperature invariable. The quantity
of outside heat for substance N 1 is DQ1 = P*DV, for substance N 2 the quantity
of heat led outside is DQ2 = i*P*DV. But since we have at our disposal the
mechanism (it is unimportant which exactly) of leading away heat from the left
part of the cylinder to the right one, the quantity of heat which should be
brought to the solution and substance N 2 from the outside will make up DQv =
DQ1 - DQ2 and DQv* = P* (1-i)* DV. Using gas law PV= MRT, where M - the
quantity of moles of the solute let`s modify
the obtained expression for DQv: DQv = P* (1-i)* DV = (1-i)* DM*RT,
where DM - the quantity of moles of solute substance N 1. We have an absolute
right to do that as movement of the
piston is accompanied by the advent of the volume of solvent which is necessary
for dilution of the next portion of the substance. However the same quantity of
moles is separated from the solution of substance N 2. So the quantity of work
which can be received during the movement of the membrane piston DA = DQv =
(1-i)* DM*RT. If the membrane is directed from left to right forcedly, then all
expended work will be educed in the form of heat on account of the same
equality. Now let`s pay attention that DM is the value depending on
temperature. It increases with the rise of temperature as solubility goes up.
Let`s consider that direct motion is fulfilled at the temperature Tmax and
return motion - at the temperature Tmin
after the cylinder is cooled up to the temperature Tmin. The problem of
prevention of heat loss in the process of cooling will be viewed a little
later. Let`s find ideal efficiency of the engine
based on direct and reverse osmosis.
A = Qvmax-Qvmin
K=
(Qvmax-Qvmin)/ Qvmax.
Opening the expression for efficiency
we`ll get the following:
or differently
Where:
DMmax - solubility at the highest possible temperature of the cycle Tmax.
DMmin - solubility at the minimal temperature of he cycle Tmin.
It
is evident that this efficiency is substantially higher than efficiency of the
ideal Carno cycle as the relation DMmin/DMmax can lie in the range (0.8 -
0.1) and it depends on concrete substances and temperature span. Compare with Carno
efficiency.
It would be interesting to value the change of
entropy of the system consisting of the concerned engine and a classical heater
and refrigerator . It is quite evident that entropy decreases in the process of
work of this engine DS = (1-i)* R* (DMmin - DMmax). There is no necessity to cite the
development of the formula on account of its evidence as DQ = (1-i)* DM*RT and DS = DQ/T. We`ll try to
explain overcoming of Carno efficiency in generally accepted terms.
As during formation of the colloidal solution a
part of thermal energy is spent on formation of the surface of micelles then
according to the first law of thermodynamics DQ= DU + PosmDV + DAs, where DAs - work on forming the surface, DU - change of
internal energy, PosmDV - in our case work of osmotic pressure. But
then entropy of formation the surface depends on Т this way: DSs =DAs/T. And since
surface tension always works for decreasing its energy then upsetting the
balance of the solution will lead to spontaneous liberation of heat
accumulated in the energy of surface tension without applying outside work
against surface tension force. In our case outside work will be spent on
overcoming only osmotic pressure-PosmDV with the purpose of achieving displacement of
dynamic equilibrium in the solution.
So roughly accepting with a view to demonstrate DAs = const, total
change of entropy will make up DSssumm = DAs*(1/Tmin-1/Tmax). In other words during the
process of work of such engine an impression will arise that entropy of
actuating fluid increases infinitely though in reality this part of joint
entropy of the process will be simply annulled by spontaneous process of
micelles. We`ll notice that any characteristic of the solution will be cycled
in the process of work of the engine in complete compliance with energy
conservation law and entropy in classical understanding must be single-valued
function of the state. As a result it becomes impossible to build up a closed
T-S diagram for thermodynamic cycle of such heat engine in classical, usual for
us style.
Probabilistic approach to explanation of this
situation is possible. In the saturated solution some micromass of the soluble
substance at the interface substance - solution has equal probability to be
found in the state of micelle and in common mass of the soluble substance
beyond the interface as the state of the saturated solution is the state of
dynamic equilibrium. If the volume of the solution increases as a result of a
certain process (in our case because of influx of the solvent through the
membrane), then probability of transmission of the substance to the solution
will be higher than probability of inverse process. In a similar manner in the
case of outflow of the solvent through the membrane, the probability of the
microvolume of the soluble substance to be found beyond the interface will be
higher than probability of transmission into the solution. Therefore performing
work against osmotic pressure it is possible to control the probability of the
processes and consequently of entropy if we proceed from boltzmann approach to
the notion of entropy.
Now let`s consider the question of conservation
of thermal energy in the process of cooling the cylinder.
In general it is possible to imagine a certain
connected conveyor from microcylinders exchanging heat. Let the membranes in microcylinders be
deprived of motion in a certain way during the process of heating - cooling and
let them go only at the extreme temperature points where work is performed by
forces of osmotic pressure or against these forces. As heats of dilution and
heat capacities of the contents of cylinders are equal and work in the process
of heating or cooling is not performed, during heat exchange on counter-current
flows consisting of microcylinders there are no heat losses and the cycle is
closed. That is to say there is no disbalance in the process of heat exchange
between cylinders because of complete energy identity of the solutions; in
other words the balance is kept in enthalpies. But technically more realistic
conceptions can be suggested. One of the possible variants is represented in
picture N6.
At upper right
there is a capacity with the membrane in which substance N 1 is dissolved and
in which from the left the solvent comes in from the cavity with a pure solvent
where its pressure is minimal. The solvent comes through the semipermeable
membrane. A transparent arrow on the scheme marks the movement of the solvent.
The solution of substance N 1 produces maximal osmotic and hydraulic pressure in
the whole system. The heat is led both from the outside and from the capacity
where substance N 2 is isolated from the solution and it is indicated by arrows
and by the sign Q. Then the saturated
solution of substance N 1 is fed to the heat exchanger where it is cooled and
part of the substance is isolated from the solution because temperature balance
of the solution is upset. The cold saturated solution of substance N 1 along
with crystals of the same substance gets into the right side of the block with
the common membrane. It is a cold block and the temperature of the cycle is
minimal here. As hydraulic pressure in this capacity is equal to the pressure
in the hot block and the osmotic pressure is much less, the solvent passes on
to the left side of the cold block with the common membrane as it is shown by a
transparent arrow. At the same time substance N 1 is isolated from the solution
and crystallization heat is transmitted to the left half of the block with the
common membrane and partly it is thrown off outside. Then substance N 1 (it is
heated up during passing) through the heat exchanger is fed to the hot block
with the membrane. As both examined
flows going through the heat exchanger moves between capacities with equal
hydraulic pressure, in this concerned ideal case we neglect work spent on it
because of its evident smallness.
Now let`s return to the cold part of
the cycle. So the solvent moves from the right to the left part of the cold
block with the common membrane. Here the hydraulic pressure is less than in the
right part, however a certain increasing of the hydraulic pressure is produced
due to osmotic pressure of substance N 2 dissolving in saturation mode.
Dissolving of substance N 2 requires some quantity of heat that is supplied by
substance N 1 isolated from the solution on the right. Then the saturated
solution of substance N 2 with the necessary quantity of substance N 2 is fed
to the heat exchanger where the solution is heated up and all substance N 2
moving along with the solution is dissolved. Heat efficiencies of dilution of
substance N 2 and isolation from the solution of substance N 1 in the heat
exchanger are equal, heat capacities and masses of substances and solutions are
equal, that`s why heat balance in the heat exchanger is kept and it is equal to
zero. As a matter of fact the heat exchanger of this engine plays a part of a peculiar
gate, that`s why it should
be as perfect as possible. Then in the hot block with the membrane flow-over of
the solvent into the part of the capacity occupied with the pure solvent takes
place as the result of hydraulic pressure difference. This flow-over occurs
against the forces of osmotic pressure, that`s why the pressure of the solvent
in the capacity with pure solvent is less than pressure of the solution but yet
it is not large enough. Upsetting the balance of the solution leads to
isolation of substance N 2. The process is accompanied by heat release utilized
by dissolving substance N 1. Received at some quite large pressure the pure
solvent is fed to the entrance of the water turbine that sets in motion the
generator. Then at the minimal pressure the solvent is fed to the fore-membrane
cavity of the block where substance N 1 is dissolving. In such a way a closed
thermodynamic cycle is generated and efficiency of it is higher than “maximum”
Carno efficiency. The substance will certainly strive for diffusion along the
pipes of the heat exchanger but diffusion rate is small in comparison with rate
of motion of liquid and that is why this factor may be neglected.
It may be shown that the engine of
such type may be built up for substances line of dissolubility of which do not
necessarily coincide precisely. Besides it may be proved that it is not
obligatory to use substances forming exactly colloidal solutions.
It is evident that suggested scheme
is easily transformed into effective refrigerator unit or thermocompressor by
means of simple selection of another pair of substances with one basic
characteristic - equal osmotic pressure and different heat of dilution.
PERFORMANCE ATTRIBUTES
Engines based on osmotic technologies will not be compact and light, large area of membranes will be required, though membranes should be laid as compact as possible. Constructions will have to stand large pressures and it means they will be thick-walled; good heat insulation will be required. A special block of purification in order to keep the solvent pure will be required as all the membranes will be permeable for soluble substances to this or that extent. It all means that such engine will have large weight falling per unit of power output. It will be possible to place such energy installation on a merchant ship and certainly in a stationary land-based form. It will hardly be possible to achieve complete identity of solutions though there may be no fundamental obstacles. However all these drawbacks of the engines will generously be repaid at today`s superhigh prices of energy resources.
CONCLUSION
We have every reason to believe that tandem of
the osmotic engine and the thermocompressor is able to build up the engine and
with maximum ideal efficiency 100 percent.
There appears a
theoretical possibility to build up the engine that does not require a
high-temperature heat source and a low-temperature refrigerator. And it will be an alternative path and it
will be greatly distinct from the traditional way in energetics.
14 March, 2008 stream_a@mail.ru Pelipenko A.I., engineer
kolis-33@yandex.ru Kolisnichenko N.D., engineer